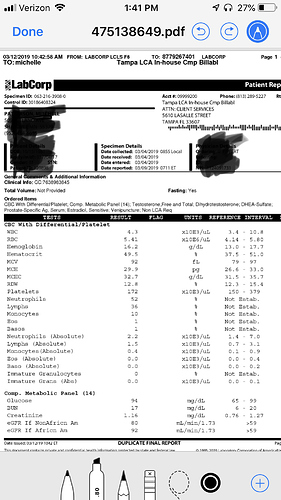
So above are my labs, my protocol is 50mg test cyp 2x a week and 1/4 anastrozle at injection… was feeling blah when labs were taken, stopped AI and upped t injections to 60mg… been super tired the last week, but also the parent of a 2 year old. Pre T my test was <300 and e2 standard was 24… now either I am an over responder or the standard test over estimated my e2… hoping I feel better in a couple more weeks, been off the AI for 4 injections… Do I need to worry about E2 rebound, is it normal to feel tired when the hormones are adjusting, even possibly in a positive way. Should I trust the labcorp 8.5 or have others had errors in their testing? My doc thought for sure I would have high E2 not low
The LC/MS/MS methodology is known to be inconsistent, but how you are feeling is more important than the number. I would let E2 elevate until if and when you had symptoms. Your dose of 50mg 2x/week is not very much and you really should not have high E2 symptoms with that protocol. I think increasing to 60mg dosing was a good idea.
Thanks
Thanks for the reassurance! So, you trust the standard test over the sensetive test? Next time I am doing both… lol
I do both, more for fun and out of curiosity than anything else.
ECLIA vs LC/MS/MS TESTING FOR E2
The ECLIA test (aka immunoassay or IA) for E2 management is commonly used for those on TRT. It is not an incorrect test or a test for women, but simply one way to check estradiol levels. The other commonly utilized test is the LC/MS/MS method (aka liquid chromatography dual mass spectrometry, sensitive or ultrasensitive). It is the more expensive of the two. There are inherent advantages and disadvantages to each of these two methods. I have been fortunate to be able to speak with professionals who work with both methods. One is a PhD researcher for Pfizer and the other is a medical doctor at Quest. I’ll summarize their comments.
The ECLIA method is the more reliable of the two in terms of consistent results. The equipment is easier to operate thus accuracy is less reliant on the skill of the operator. If the same sample were to be tested twenty times, there would be very little, if any, difference in the results.
The ECLIA method is not as “sensitive” in that it will not pick up E2 levels below 15pg/mL. If your E2 level with this test is 1-14pg/mL, the reported result will be “<15”. Because of this, it is not recommended for menopausal women, men in whom very low levels of E2 are suspected, or children. In other words, if your levels are below 15pg/mL, and it is important to know if the level is 1 or 14pg/mL, you do not want this test. For us, this is likely moot, since if you are experiencing low E2 symptoms and your test comes back at <15, you have your answer. For a woman being treated with anti-estrogen therapy for breast cancer, it may be necessary to know if the E2 level is zero or fourteen because therapeutically, they want zero estrogen.
A disadvantage to IA testing is that it may pick up other steroid metabolites, which in men would be very low levels, but still could alter the result. Another potential disadvantage is that elevated levels of C-reactive protein (CRP) may elevate the result. CRP is elevated in serious infections, cancer, auto-immune diseases, like rheumatoid arthritis and other rheumatoid diseases, cardiovascular disease and morbid obesity. Even birth control pills could increase CRP. A normal CRP level is 0-5 to 10mg/L. In the referenced illnesses, CRP can go over 100, or even over 200mg/L. Unless battling one of these serious conditions, CRP interference is unlikely.
The LC/MS/MS method will pick up lower E2 levels and would be indicated in menopausal women and some men if very low E2 levels are suspected and it is desired to know exactly how low, children and the previously mentioned women on anti-estrogen therapy. It will not be influenced by elevated CRP levels or other steroid metabolites.
While some may believe the ECLIA test is for women, on the contrary, as it pertains to women on anti-estrogen therapy, such as breast cancer patients, the LC/MS/MS is the test for women as CRP levels are a consideration and it is necessary to know if the treatment has achieved an estrogen level of zero.
On the other side of the coin, LC/MS/MS equipment is “temperamental” (as stated by the PhD who operates both) and results are more likely to be inconsistent. Because of this, researchers will often run the same sample multiple times.
It is not clear if FDA approval is significant, but this appears on Quest’s lab reports: This test was developed, and its analytical performance characteristics have been determined by Quest Diagnostics Nichols Institute San Juan Capistrano. It has not been cleared or approved by FDA. This assay has been validated pursuant to the CLIA regulations and is used for clinical purposes. This statement is on LabCorp’s results: This test was developed and its performance characteristics determined by LabCorp. It has not been cleared by the Food and Drug Administration.
It is unlikely that any difference in the same sample run through both methods will be clinically significant. Estradiol must be evaluated, and it should be checked initially and ongoing after starting TRT. It obviously makes sense to use the same method throughout. Most important are previous history and symptoms related to low or high E2. Those are correlated with before and after lab results. Any estradiol management should not be utilized without symptoms confirmed by lab results.